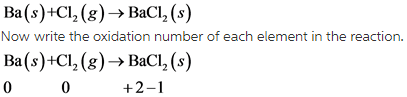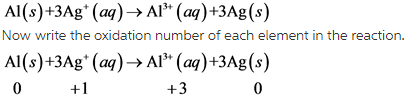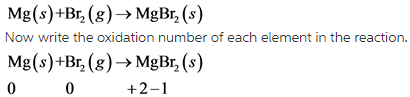13
Q:
| A) 1 | B) 2 |
| C) 3 | D) 4 |
Answer: D) 4
Explanation:
Explanation:
Here all the given options are Exothermic reactions. Exothermic reactions are those reactions which involves the release of heat.
Generally all the combining reactions are exothermic in nature.

 Aptitude and Reasoning
Aptitude and Reasoning General Knowledge
General Knowledge Puzzles
Puzzles Interviews
Interviews Technical
Technical Certifications
Certifications Exams
Exams Job
Roles
Job
Roles True or False
True or False Exams
Exams