Q:
Which of these molecules are polar? Check all that apply.
Answer & Explanation
Answer: D) All the above
Explanation: 1) SO2 2) CH2Cl2 3) PCl3
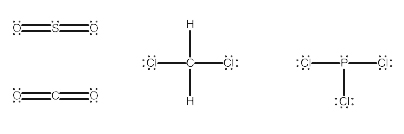
How to check the given molecules are polar or non polar :
If the electronegativity of the two bonded atoms are different then the bond is polar. Although, some molecules contain polar bonds, the molecule may be non-polar. If the polarity of all the bonds present in the molecule are cancel each other then molecule is non-polar.
In the given options,
SO2 is polar because the dipole moments on the O do not cancel.
PCl3 is polar because the P-Cl bonds do not cancel out.
CH2Cl2 polar because the C-Cl bonds do not cancel.
Hence, in brief, Polar molecules are molecules with net dipole due to the presence of partial positive and partial negative charge.
For example, water is a polar molecule that has partial positive and partial negative charge on it. CO2 is a non polar compound.
Therefore, all the given options SO2, CH2Cl2 and PCl3 are Polar molecules.
View Answer
Report Error
Discuss